
Koselugo delivered a strong response with evidence from long-term data1,6††
View the long-term safety profile here.

ORR was defined as the percentage of patients with complete response (defined as disappearance of the target PN) or confirmed partial response (defined as ≥20% reduction in PN volume confirmed at a subsequent tumor assessment within 3 to 6 months).1†
An independent centralized review (ICR) of tumor response per REiNS criteria resulted in an ORR of 44% (95% CI: 30, 59).1
*DCO June 2018.1
†33 partial responses were confirmed by 3D MRI volumetric analyses at a subsequent tumor assessment within 3 to 6 months. The ORR assessment was conducted by a single NCI reviewer who was a SPRINT investigator and who evaluated all PN imaging from patients enrolled at all trial sites.1
MOA=mechanism of action; PN=plexiform neurofibromas.

‡Koselugo is the first FDA-approved therapy for pediatric patients with NF1 who have symptomatic inoperable PN.1,2
§DCO June 2018.3

DOR was defined as the time from the pre-cycle volumetric MRI assessment of the first documented response (which was subsequently confirmed) until the pre-cycle volumetric MRI assessment of documented progression.5
¶DCO March 2021.1
#Median DOR was not reached (95% CI: 41.2 months, NE).1
||DCO February 2021. This information is from the SPRINT long-term follow-up study.4
CI=confidence interval; DCO=data cutoff; DOR=duration of response; FDA=Food and Drug Administration; MRI=magnetic resonance imaging; NCI=National Cancer Institute; NE=not evaluable; NF1=neurofibromatosis type 1; ORR=overall response rate; REiNS=Response Evaluation in Neurofibromatosis and Schwannomatosis; 3D=three dimensional.
Before Koselugo

After Koselugo

Based on an AstraZeneca analysis of NCI data6
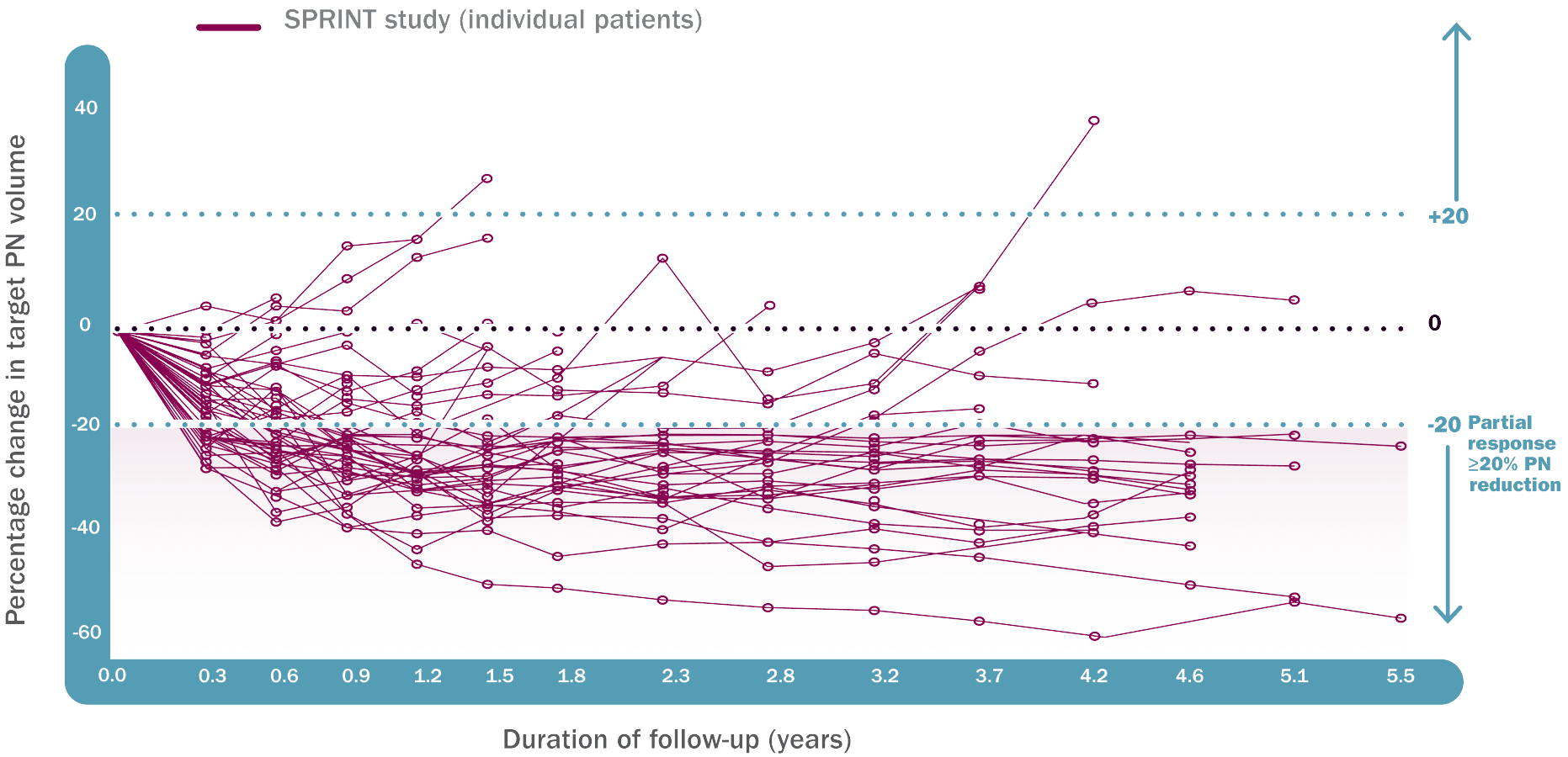
**DCO March 2021.6
See study design
View the long-term safety profile here.
††At DCO June 2018, 66% (33/50) achieved ≥20% tumor reduction. A subsequent long-term follow-up study was included (DCO February 2021).1,4

‡‡The median time to onset of response was 7.2 months. One more patient achieved ≥20% PN shrinkage after 1.6 years.1
§§DCO: March 2021 where there were 34 responders, compared to the 33 responders at the June 2018 DCO.3,6
¶¶Progression-free survival (PFS) was calculated as the number of cycles of treatment until disease progression or censoring at the time of coming off treatment or most recent restaging evaluation.5
##PFS had a DCO of March 2021 and was calculated using Kaplan-Meier method.1,5
BID=twice daily.
Time to onset of response among patients who responded to Koselugo3

||||The median time to onset of response was 7.2 months (range: 3.3 months to 1.6 years).1
***DCO June 2018.3
†††A cycle was defined as 28 days.5
Cardiomyopathy. A decrease in left
ventricular ejection fraction (LVEF) ≥10% below baseline occurred
in pediatric
patients who received Koselugo in SPRINT with some
experiencing decreased LVEF below the institutional lower limit of normal
(LLN), including one patient with Grade 3. All patients with decreased
LVEF were asymptomatic and identified during routine echocardiography.
The safety of Koselugo has not been established in patients with a history
of impaired LVEF or a baseline ejection fraction that is below the institutional
LLN. Assess ejection fraction by echocardiogram prior to initiating treatment,
every 3 months during the first year of treatment, every 6 months thereafter,
and as clinically indicated. Withhold, reduce dose, or permanently discontinue
Koselugo based on severity of adverse reaction. In patients who interrupt
Koselugo for decreased LVEF, obtain an echocardiogram or a cardiac MRI
every 3 to 6 weeks. Upon resolution of decreased LVEF, obtain an echocardiogram
or a cardiac MRI every 2 to 3 months.
Ocular Toxicity. Blurred vision,
photophobia, cataracts, and ocular hypertension
occurred. Retinal
pigment epithelial detachment (RPED) occurred in the pediatric population
during treatment with single agent Koselugo and resulted in permanent discontinuation.
Conduct ophthalmic assessments prior to initiating Koselugo, at regular
intervals during treatment, and for new or worsening visual changes. Permanently
discontinue Koselugo in patients with retinal vein occlusion (RVO). Withhold
Koselugo in patients with RPED, conduct ophthalmic assessments every 3
weeks until resolution, and resume Koselugo at a reduced dose.
Gastrointestinal Toxicity. Diarrhea
occurred, including Grade 3. Diarrhea resulting in permanent discontinuation,
dose interruption or dose reduction occurred. Advise patients to start
an anti-diarrheal agent (eg, loperamide) and to increase fluid intake immediately
after the first episode of diarrhea. Withhold, reduce dose, or permanently
discontinue Koselugo based on severity of adverse reaction.
Skin Toxicity. Rash occurred in 91% of 74 pediatric patients. The most frequent rashes included dermatitis acneiform (54%), maculopapular rash (39%), and eczema (28%). Grade 3 rash occurred, in addition to rash resulting in dose interruption or dose reduction. Monitor for severe skin rashes. Withhold, reduce dose, or permanently discontinue Koselugo based on severity of adverse reaction.
Increased Creatine Phosphokinase (CPK). Increased CPK occurred, including Grade 3 or 4 resulting in dose reduction. Increased CPK concurrent with myalgia occurred, including one patient who permanently discontinued Koselugo for myalgia. Obtain serum CPK prior to initiating Koselugo, periodically during treatment, and as clinically indicated. If increased CPK occurs, evaluate for rhabdomyolysis or other causes. Withhold, reduce dose, or permanently discontinue Koselugo based on severity of adverse reaction.
Increased Levels of Vitamin E and Risk of Bleeding. Koselugo capsules contain
vitamin E which can inhibit platelet
aggregation and antagonize vitamin K-dependent clotting factors. Supplemental
vitamin E is not recommended if daily vitamin E intake (including the amount
of vitamin E in Koselugo and supplement) will exceed the recommended or
safe limits due to increased risk of bleeding. An increased risk of bleeding
may occur in patients who are coadministered vitamin-K antagonists or anti-platelet
antagonists with Koselugo. Monitor for bleeding in these patients and increase
international normalized ratio (INR) in patients taking a vitamin-K antagonist.
Perform anticoagulant assessments more frequently and adjust the dose of
vitamin K antagonists or anti-platelet agents as appropriate.
Embryo-Fetal Toxicity. Based on
findings
from animal studies, Koselugo can cause fetal harm when
administered during pregnancy. In animal studies, administration of selumetinib
to mice during organogenesis caused reduced fetal weight, adverse structural
defects, and effects on embryo-fetal survival at approximate exposures
>5 times the human exposure at the clinical dose of 25 mg/m2 twice daily.
Advise patients of reproductive potential of the potential risk to a fetus
and to use effective contraception during treatment with Koselugo and for
1 week after the last dose.
Common adverse reactions ≥40% include vomiting, rash (all), abdominal pain, diarrhea, nausea, dry skin, musculoskeletal pain, fatigue, pyrexia, acneiform rash, stomatitis, headache, paronychia, and pruritus.
Effect of Other Drugs on Koselugo
Concomitant use of Koselugo with a strong or moderate CYP3A4 inducer decreased selumetinib plasma concentrations, which may reduce Koselugo efficacy. Avoid concomitant use with Koselugo.
Pregnancy & Lactation. Verify the
pregnancy status of patients of reproductive potential prior to
initiating Koselugo. Due to the potential for adverse reactions in a breastfed
child, advise patients not to breastfeed during treatment with Koselugo
and for 1 week after the last dose.
KOSELUGO is indicated for the treatment of pediatric patients 2 years of age and older with neurofibromatosis type 1 (NF1) who have symptomatic, inoperable plexiform neurofibromas (PN).
To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca
1-800-236-9933
or at
https://us-aereporting.astrazeneca.com
or FDA at
1-800-FDA-1088 or
www.fda.gov/
medwatch.
Please see full Prescribing Information for Koselugo® (selumetinib).
1. Koselugo. Package insert. AstraZeneca Pharmaceuticals LP.
2. Koselugo (selumetinib) approved in US
for paediatric patients with neurofibromatosis type 1 plexiform
neurofibromas. AstraZeneca. April 13, 2020. Accessed December 4, 2023. https://www.astrazeneca.com/media-centre/press-releases/2020/koselugo-selumetinib-approved-in-us-for-paediatric-patients-with-neurofibromatosis-type-1-plexiform-neurofibromas.html#
3. Data on File, REF-75729, AstraZeneca Pharmaceuticals LP.
4. Gross AM, Dombi E, Wolters PL, et al. Long-term safety
and efficacy of selumetinib in children with neurofibromatosis type 1 on
a phase 1/2 trial for inoperable plexiform neurofibromas. Neuro Oncol. 2023;25(10):1883-1894. doi:10.1093/
neuonc/noad086
5. Data on File, REF-36656, AstraZeneca Pharmaceuticals LP.
6. Data on File, REF-36657, AstraZeneca Pharmaceuticals LP.